SIMSTECH's 'Dr. Tracking' is a digital reusable surgical instruments management system that
includes an entire business process, which automatically records usage history of a series
of infection prevention processing tasks for each instrument, in order to ensure that
patients in hospitals are safe from any possible medical infections.
‘Dr.Tracking’ ensures transparency that reusable surgical instruments required by all
departments within a hospital can be delivered accurately and quickly at the right time and
place with getting rid of any infection risk.
Under laws globally, it is a requirement that all reusable medical devices used on a patient
are sterile.
All reusable medical devices must be traceable as to when, how, and by whom they were used.
This is because if an infection occurs in a patient after surgery, the hospital must provide
evidence that each device has undergone an appropriate sterilization process.
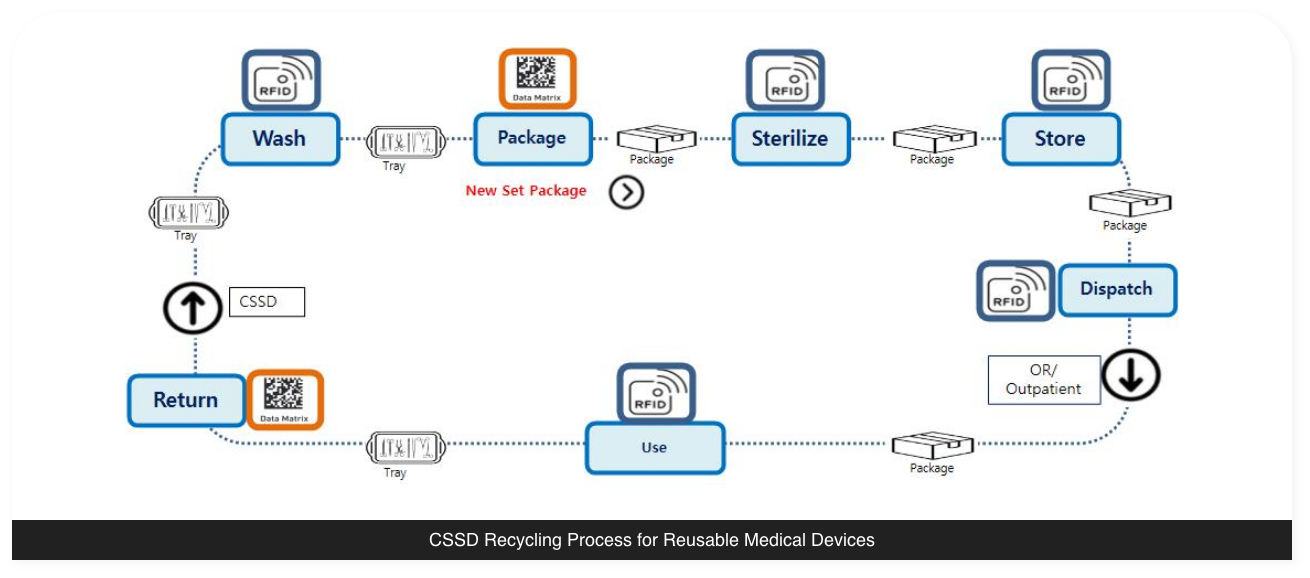
Searching through paper forms is a quite difficult task. Instead, ‘Dr.Tracking’ makes it
possible to directly trace for individual medical devices through the entire reprocessing
cycle starting from decontamination, cleaning, packaging, sterilization, storage, and up to
return after surgical use.




‘Dr.Tracking’ enables complete traceability management of reusable medical devices, which results in increased work efficiency in the event of an emergency and ultimately regulatory compliance for medical infection prevention.
'Dr.traking' mainly consists of 3 following technologies and its systems
01
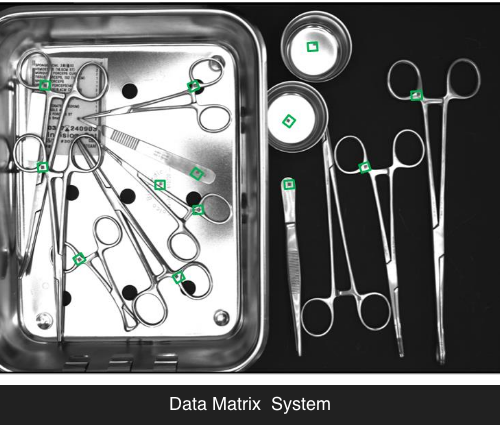
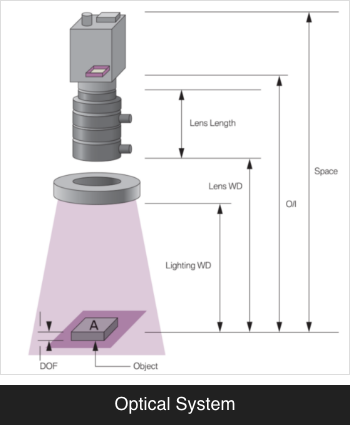
UDI (Unique Device Identifier) Marking System

02
UDI (Unique Device Identifier) Analysis System
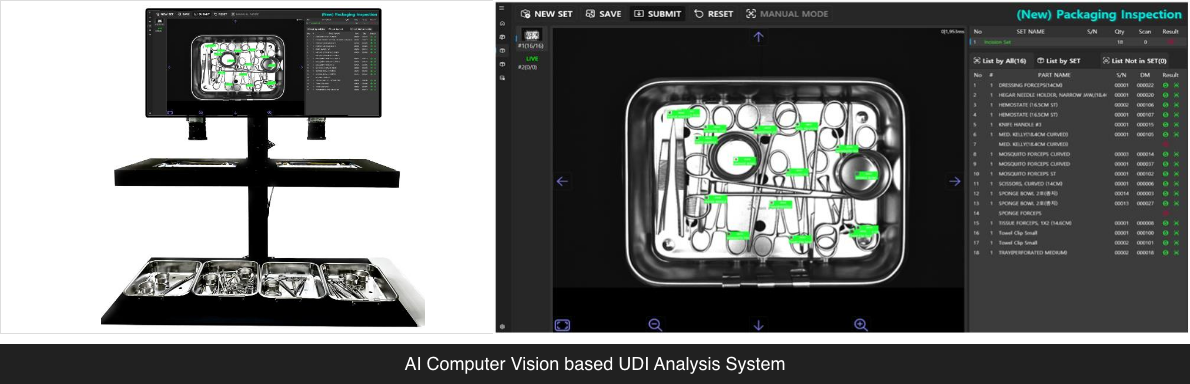
03
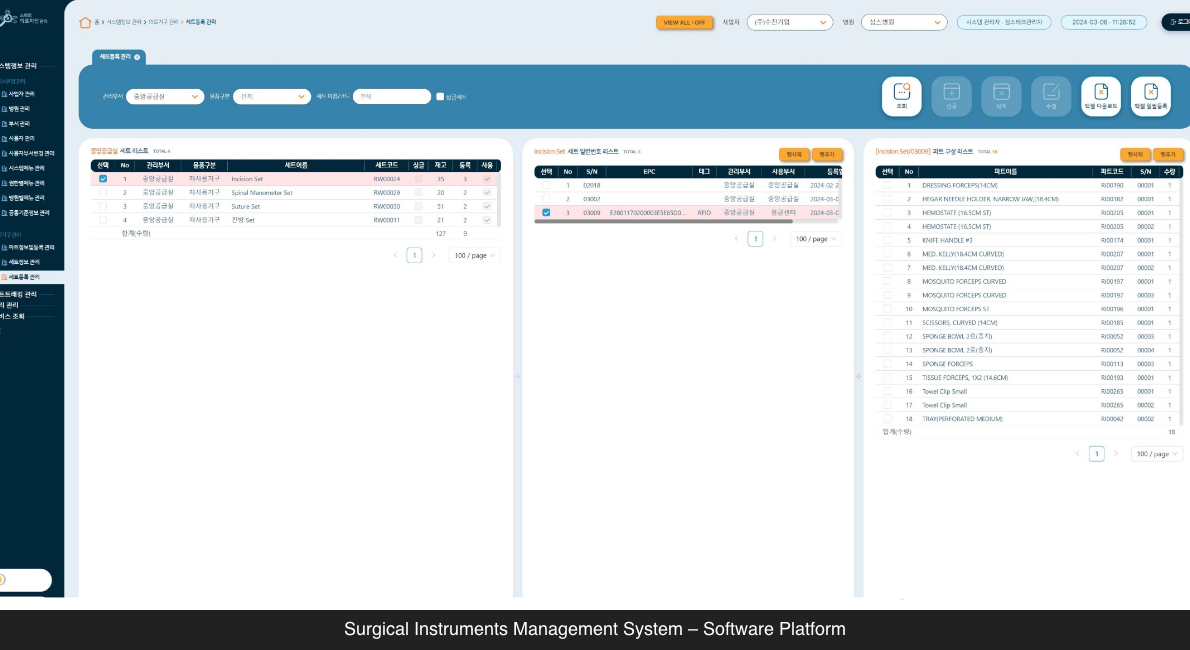
Surgical Instruments Management System – Software Platform
Identification of reusable medical devices
Assigning a unique identification code with 2D Data Matrix,
each medical device can be identified and its location can be tracked in real time.
01
Real-time Emergency Tracking
By tracking the patient-related usage history of each medical device,
you can respond quickly if a infection problem arises.
02

Real-time Inventory Management
Track and manage the inventory status of medical devices in real time.
If necessary, order and stock them to prevent inventory shortages.
03
Maintenance management
Track and Manage the maintenance status of each medical device.
This maintains the performance of medical devices and ensures safety.
04
Notification and warning
Provides notifications about important safety matters such as the expiration date of
each medical device. This allows medical
staff to respond to medical risks in a
timely manner.
05

Reports and analysis
Analyze usage history and maintenance-related data to identify opportunities
for improving inventory efficiency and cost.
06
01
RFID

02
Data Matrix